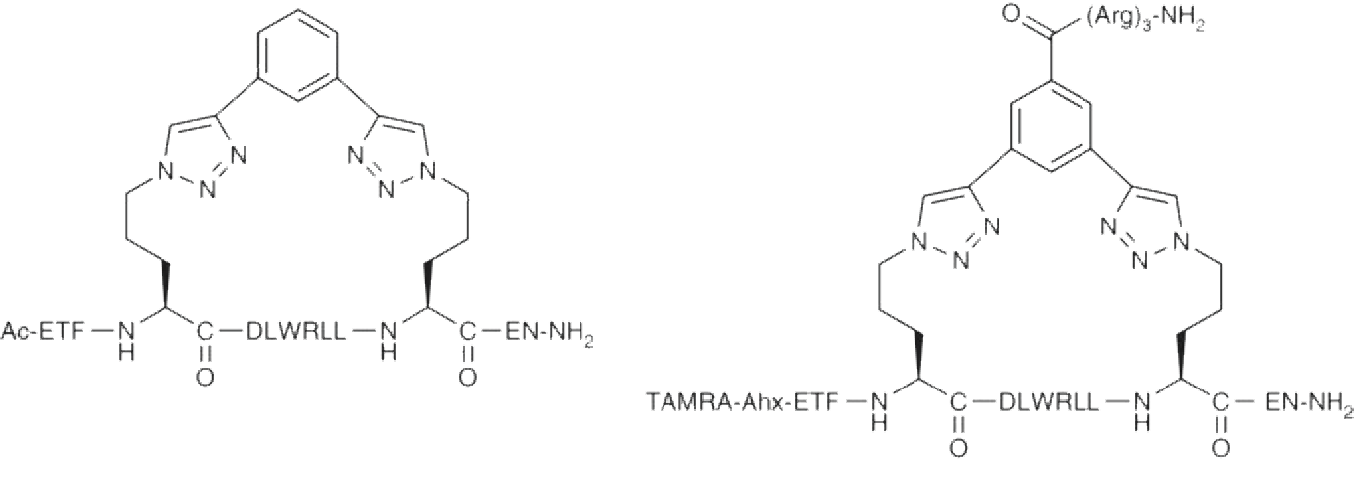
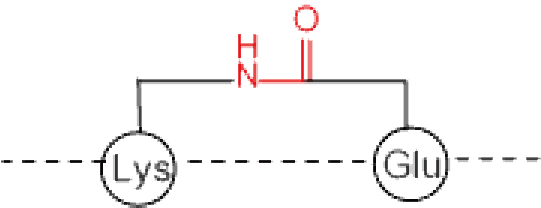Cyclic Peptide Synthesis
Peptide cyclization is carried out on the backbone or side chain of the peptide. It not only eliminates the amino and carboxyl groups at the N-terminus and C-terminus of the peptide that are susceptible to degradation by exopeptidases, but also enables the peptide to maintain a conformation that is conducive to binding to the receptor, thereby greatly improving the biological activity of these peptides.
Cyclic Peptide Types
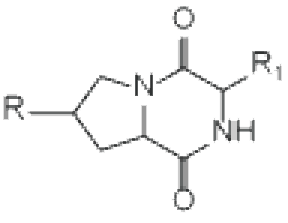
cyclo(X1-X2——–Xn) Head-to-tail cyclization | Diketopiperazine | |
|
| 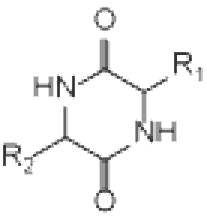 |
X = NH : polypeptide X = O : depsipeptide (including N-methyl amino acids) | R=H : cyclo(X-Pro) R=OH : cyclo(X-Hyp) | cyclo(X-X) |
Cyclization at side chain | Lactone/Thiolactone | Thioether |
| 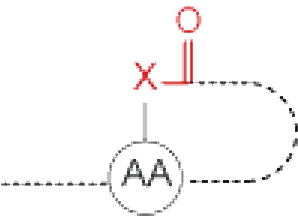 | 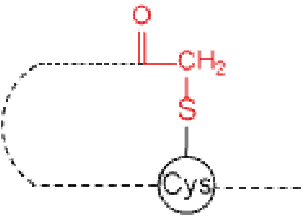 |
X=O : lactone X=S : thiolactone | ||
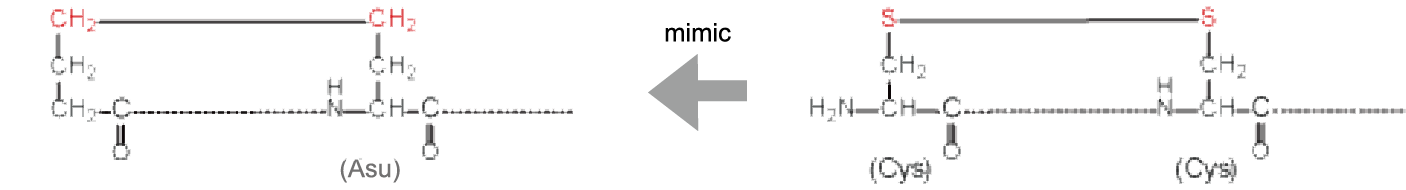
Deamino-dicarba | ||
| ||
Stapled Peptides | ||
| ||
Stapled peptides are modified peptides that usually have an α-helical conformation and are constrained by a synthetic scaffold (staple compound). Stapled peptides have a higher degree of α-helical conformation and significantly enhance their ability to bind to targets. They can penetrate cell membranes, are difficult to be hydrolyzed by proteases, and have a longer half-life in vivo. | ||